Introduction
Cardiac magnetic resonance imaging (MRI) is a noninvasive and reliable imaging tool, which provides a comprehensive examination of cardiac morphology, functions, and disorders [1]. The absence of ionizing radiation, high soft-tissue contrast, and multiplanar evaluation are the main advantages of cardiac MRI [1, 2]. Cardiac MRI has become the standard non-invasive method for evaluating cardiac functions [1-3]. Furthermore, quantitative T1, T2, and T2* mapping have recently achieved clinical utility in numerous myocardial pathologies such as myocardial fibrosis, myocarditis, amyloidosis, and thalassaemia because they provide non-invasive tissue characterization with the potential to replace myocardial biopsy [4]. Therefore, the use and need for cardiac MRI is increasing day by day.
Outside the heart, a significant part of the body (breast, lung, and upper abdomen) is usually also screened in each cardiac MRI examination. Thus, major pathologies in other organs such as lung, mediastinum, liver, or breast can be incidentally detected and may have an impact on further patient prognosis and management. Therefore, major non-cardiac findings (NCFs) should be investigated and reported when evaluating cardiac MRI examinations. In the literature, the prevalence of discovered NCFs during routine cardiac MRI is highly variable and ranges from 7.6% to 81.0% [5]. Those studies in the literature were about the frequency of NCF in official cardiac MRI reports, and it is estimated that the second reading may detect higher NCF than the first reading. However, to the best of our knowledge there is no study in the literature on how often NCFs are reported in official radiology reports and the frequency of unreported pathologies. Therefore, our aim was to evaluate the prevalence and significance (insignificant, significant, or major) of NCFs in patients who underwent cardiac MRI. We also aimed to assess the rate and clinical significance of unreported NCFs in archived official radiology reports. Thus, we aimed to demonstrate the differences between the official cardiac MRI reports and secondary readings in terms of NCFs. We also aimed to assess the clinical significance of these reading differences.
Material and methods
This retrospective study was approved by the institutional review board. All consecutive cardiac MRI examinations between July 2017 and August 2020 were retrospectively analysed. All cardiac MRI examinations were obtained based on Society for Cardiovascular Magnetic Resonance recommendations [6].
All cardiac MRI images were acquired using a 1.5-Tesla MRI scanner (Philips Ingenia, Philips Healthcare, USA) and a torso cardiac coil (dStream, Philips Healthcare, USA). After 3-plane (axial, coronal, and sagittal) localizer images, axial and coronal balanced steady-state free precession (b-SSFP) sequences were acquired during a single breath-hold through the chest, respectively. Because the b-SSFP sequence de-monstrates the cardiac and cardiovascular anatomy well, it is always obtained in the cardiac MR examinations in our centre. The acquisition time was about 25-35 seconds for each plane. b-SSFP sequence parameters were as follows: field of view (FOV): 30 cm, repetition time (TR): 2.5 msec, echo time (TE): 1.2 msec, turbo factor: 70, slice thickness: 7 mm, interslice gap: none, and number of signals averaged (NSA). Afterward, for a detailed evaluation of the cardiac chambers, cardiovascular and chest morphology axial half-Fourier acquisition single-shot turbo spin-echo (SSH-TSE) sequence with double inversion recovery (also known as black blood or dark blood technique) were obtained from the lung apex to the upper abdomen during free-breathing in all patients. Dark blood technique is a successful and recommended technique, especially in the evaluation of congenital heart diseases, vascular anomalies, and cardiac masses [6]. In the dark blood technique, we obtain the images from the entire thorax in our cardiac MRI protocol to evaluate possible mediastinal and lung lesions. The acquisition time of SSH-TSE sequence was about 80-90 seconds, and imaging parameters were as follows: field of view (FOV): 30-35 cm, repetition time (TR): 2222 msec, echo time (TE): 31 msec, turbo factor: 50, slice thickness: 6 mm, interslice gap: 1 mm, and number of signals averaged (NSA). The MRI examination was then completed according to clinical suspicion with the appropriate sequences as specified in the guidelines [6].
Blinded to official reports of the cardiac MRI examinations, 2 radiologists with 4 and 19 years of experience, respectively, in cardiothoracic imaging reviewed all b-SSFP and black blood images to investigate the presence of NCF. Furthermore, NCFs were classified as insignificant (which has no clinical significance, and treatment is not required, such as right aortic arch), significant (which has clinical significance, and follow-up is required, such as mild ascending aorta dilatation, between 41 and 49 mm), and major (which has clinical significance, and further evaluation or treatment is required, such as lung mass), as described by Mantini et al. [7]. In patients with significant and major findings, NCFs were classified as previously known or unknown, based on the clinical archive. Furthermore, we compared our systematic approach with the archived official cardiac MRI reports as it was used for reporting NCFs to estimate unreported rates. The patients who had major NCF and were clinically followed up after MRI examination were investigated from the clinical archive.
The (insignificant, significant, and major) prevalence of NCFs and the number of previously known or unknown NCFs were evaluated. The relationship between NCFs and the patient’s characteristics (age and gender) were investigated. The differences between groups were assessed by analysis of variance or Student’s t-test, as appropriate. The relationship between age and number of NCFs was calculated by Spearman’s correlation coefficient (r). SPSS (SPSS for Windows, IBM Corp. Version 24. Armonk, NY) was used for statistical analyses, and a p-value < 0.05 was considered statistically significant.
Results
The mean age of the 400 patients (235 M; 165 F) was 46.7 ± 20.7 years (median: 48 years; range: 18-85 years). There was no statistically significant age difference between the genders (p = 0.102; 39.1 ± 19.3 years in males and 42.2 ± 17.2 years in females). The most common indication for cardiac MRI was suspected cardiomyopathies (n = 122, 30.5%), suspected cardiac mass (n = 85, 21.3%), followed by congenital heart disease (n = 72, 18.0%) and syncope (n = 50, 12.5%) (Table 1).
Table 1
Clinical indications of cardiac magnetic resonance imaging
Of 400 patients, 137 patients (34.3%) had a total of 175 NCFs, and 263 patients (65.7%) had no NCFs. Of the 137 patients, 111 had 1 NCF, 16 had 2 NCFs, 8 had 3 NCFs, and 2 had 4 NCFs. Ninety-three NCFs were considered as insignificant (53.1% of all NCFs in our cohort), 59 were significant (33.7%), and 23 (13.1%) were major (Figures 1-3). The most frequent NCFs were pleural effusion (n = 31, 17.7%), pulmonary artery (n = 15, 8.6%), and ascending aorta dilatations (n = 12, 6.9%). Of 82 significant and major NCFs, the most frequent NCFs were pleural effusion (n = 18, 22.0% of all significant and major NCFs), pulmonary artery dilatation (n = 15, 18.3%), and ascending aorta dilatation (n = 12, 14.6%) (Table 2). The median follow-up was 13 months (range; 6 to 27 months) for the patients who had major NCFs.
Figure 1
Four cases with insignificant noncardiac findings demonstrated in axial balanced steady-state free-precession images represented by: A) minimal left pleural effusion (arrow), B) thymic hyperplasia (arrow), C) persistent left superior vena cava (arrow), and D) pectus excavatum deformity (arrow)
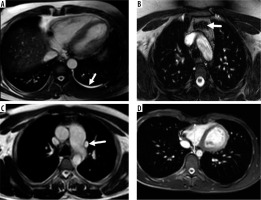
Figure 2
Four cases with significant non-cardiac findings demonstrated in axial, coronal balanced steady-state free-precession (b-SSFP), and axial half- Fourier acquisition single-shot turbo spin-echo (SSH-TSE) images represented by the following: A) perihepatic and perisplenic effusion (b-SSFP; arrow), B) enlarged mediastinal and hilar lymph nodes (SSH-TSE; arrow), C) pulmonary artery dilatation (b-SSFP; arrowheads), D) ascending aorta dilatation in a patient with a history of Senning operation due to TGA (b-SSFP; arrow)
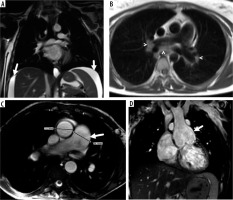
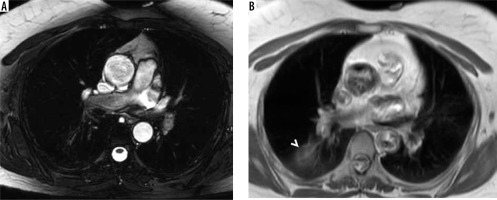
Figure 3
Four cases with major non-cardiac findings demonstrated in axial, coronal balanced steady-state free-precession (b-SSFP) and axial half-Fourier acquisition single-shot turbo spin-echo (SSH-TSE) images represented by the following: A) right lower lobe consolidation (SSH-TSE; arrowheads), B) anterior mediastinal mass compatible with thymic malignancy (b-SSFP; arrows), C) lung nodule in the right upper lobe (SSH-TSE; arrow), and D) pulmonary embolism (b-SSFP; arrow)
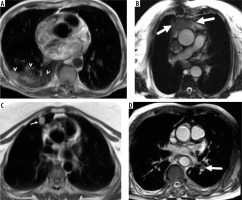
Table 2
Non-cardiac findings
Of 82 significant and major NCFs, 25 (8 of 25 were major) in 22 patients were previously unknown (Table 3). In the evaluation of patients with more than 1 NCF, a patient had moderate pleural effusion, pulmonary artery dilation, and portal vein thrombosis. Another patient had both minimal pleural effusion (< 1 cm depth in axial MRI) and hiatal hernia (Figure 4). When major NCFs were identified and officially reported at the time of the cardiac MRI, further investigation was recommended in all cases. In the evaluation of patients with previously unknown and major NCF, 1 patient with pulmonary consolidation was diagnosed with infectious pneumonia, and a suprarenal gland nodule was diagnosed as adenoma by dual-phase abdominal MRI. Two patients with pulmonary nodules (> 10 mm) underwent a CT-guided biopsy and were diagnosed with organizing pneumonia and adenocarcinoma. The diagnosis of pulmonary embolism in a patient was confirmed with pulmonary CT angiography. Although official reports describe a patient with pulmonary consolidation and another patient with a complex renal cortical cyst, there were no follow-up and further examination records in these patients’ archives. One patient with portal vein thrombosis was previously unknown and unreported in the official radiology report. In the second reading, portal vein thrombosis was detected, and the patient was called. The diagnosis of partial portal vein thrombosis was confirmed by Doppler ultrasonography. The patient was diagnosed with Behçet’s disease for 5 years, and after the diagnosis of portal vein thrombosis he was treated with enoxaparin. A 12-mm subsolid lung nodule was not reported in the official radiology report in 1 patient. The nodule was detected by chest CT examination and diagnosed as minimally invasive adenocarcinoma.
Figure 4
Axial balanced steady-state free-precession image shows minimal right pleural effusion (arrowhead) and hiatal hernia (arrow)
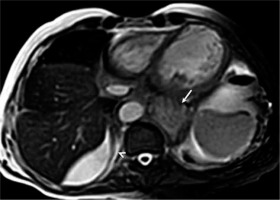
Table 3
Previously unknown significant and major non-cardiac findings
According to our classification, significant and major NCFs were reported in 41/59 (69.5%) and in 19/23 (82.6%), respectively. Pulmonary artery dilatation (ł 29 mm) was the least reported NCF (n = 7), followed by aorta dilatation (≥ 40 mm, n = 3). Other unreported findings were hiatal hernia (n = 2), pulmonary nodule (ł 6 mm, n = 2), mediastinal or hilar lymph node enlargement (ł 10 mm, n = 1), splenomegaly (n = 1), breast nodule (n = 1), portal vein thrombosis (n = 1), gallstone (n = 1), thyroid gland nodule (n = 1), pulmonary embolism (n = 1), and liver nodule (n = 1) (Table 4). The unreported liver nodule was previously known, and the nodule was colon carcinoma metastasis. In the evaluation of patients with major NCFs that were previously unknown and unreported in the official radiology report, in the patients with portal vein thrombosis and pulmonary embolism the diagnoses were confirmed with further evaluations, as described before. The unreported lung nodule was dia-gnosed with adenocarcinoma, as described above.
Table 4
Unreported findings in official radiology reports
Of the 23 major NCFs determined by the second evaluation in the cardiac MRI examination, 3 were previously unknown and were not included in the official report. Although a larger number of major NCFs were detected by the second reading, as expected, no statistical difference was found between official radiology reports and second reading for the detection of major NCFs (p = 0.083).
Patients with NCFs were significantly older than other patients (mean age, 46.7 ± 20.7 vs. 36.2 ± 17.2 years; p < 0.0001). Patients with significant and major NCFs were older than controls (mean age, 49.7 ± 21.3 vs. 37.8 ± 19.1 years; p = 0.001) and patients with insignificant NCFs (mean age, 51.9 ± 22.3 vs. 41.2 ± 18.6 years; p = 0.02). A significant positive correlation was found between age and number of NCFs (p = 0.024, r = 0.193).
Discussion
Our study demonstrated that the incidental detection of NCFs during cardiac MRI is frequent, and the vast majority of identified NCFs are clinically insignificant. The detection and clinical importance of NCFs increases with age. Although a small number of previously unknown major NCFs were identified on routine cardiac MRI (n = 8, 2% of the total population), it has a significant impact on the diagnosis and treatment of patients. Although a minority of the previously unknown major NCFs were not reported in official MRI reports (n = 4, 1% of the total population), no statistical difference was found between official MRI reports and second consensus reading for the detection of major NCFs (p = 0.083).
In the literature, the prevalence of discovered NCFs during routine cardiac MRI is highly variable and ranges from 7.6% to 81.0% [5]. Mantini et al. [7] found that 21.6% of patients (108/500) had NCF, Greulich et al. [8] found that 21.9% of patients (235/1074) had NCF, Irwin et al. [9] found that 21.6% of patients (154/714) had NCF, Wyttenbach et al. [10] reported that 48% of patients (187/393) had NCF, Atalay et al. [11] reported that 27.1% of patients (65/240) had NCF, Khosa et al. [12] reported that 43% of patients (212/495) had NCF, and Dunet et al. [13] reported that 57.7% of patients (440/762) had NCF. Moreover, the comprehensive systemic review and meta-analysis by Dunet et al. (14), which included 7062 patients, reported the total NCF prevalence as 35%. Similarly, our study showed the NCF prevalence as 34.3% (137/400). This difference in the literature is most likely due to the comorbidities and average age of the study population, distinctive FOV values, and various MRI sequences (b-SSFP, SSH-TSE, scout images) used for NCF detection. Also, the studies found in the literature made an assessment of NCF based solely on official MRI reports, and the NCF frequency was calculated using official MRI reports. As expected, our study showed that the detection prevalence of significant and major NCF is significantly higher with a second review but statistically not significant (p = 0.083). The incidence of NCF will increase in patients with comorbidities such as malignancy, and the frequency of NCF increases with age, which was demonstrated in our study. In the literature, distinctive MRI sequences were used for the detection of NCFs. Irwin et al. [9] and Dunet et al. [13] used SSH-TSE sequences, Greulich et al. [8] used SSH-TSE sequences, Wyttenbach et al. [10] used cardiac MRI sequences including scout images, and Roller et al. [15] and Mantini et al. [7] used axial b-SSFP sequences. However, we used both SSH-TSE and b-SSFP sequences for the assessment of NCF presence. Sohns et al. [16] reported that b-SSFP was more successful than SSH-TSE in the detection of NCFs. However, in our study, a nodule with histopathology of lung adenocarcinoma was not seen in the b-SSFP sequence but was clearly seen in the SSH-TSE sequence (Figure 5). Therefore, obtaining both SSH-TSE and b-SSFP sequences for the entire thorax in routine cardiac MRI exams may increase the frequency of detection of ground-glass lung nodules, which may be vital, even though they cause a tolerable elongation during the acquisition.
Figure 5
A 42-year-old female with a right lung nodule. The nodule was histopathologically confirmed as lung adenocarcinoma. While the lung nodule cannot be seen in the axial balanced steady-state free precession image (A), it is clearly visible in the axial half-Fourier acquisition single-shot turbo spinecho image (B; arrowhead)
Our data were consistent with the prevalence of significant and major NCFs reported in the literature during cardiac MRI. Mantini et al. [7] reported 94 “significant and major NCF” in 500 patients, Wyttenbach et al. [10] reported 84 “potentially significant” NCFs in 400 patients, Irwin et al. [9] reported 90 “major NCFs” in 714 patients, and Greulich et al. [8] reported 168 “major and highly relevant NCFs” in 1074 patients. Likewise, we found 82 significant and major NCFs in 400 patients in our study. However, the extensive systematic review and meta-analysis Dunet et al. divided NCFs as major and minor, and reported the major NCF frequency as 12% [14]. The prevalence of major NCFs in this study was considerably higher than our findings (5.8%). This is probably because Dunet et al. [14] divided the NCFs into just 2 groups as major and minor. For example, in that study, aortic dilatation, pulmonary artery dilatation, and pleural effusion were classified under major NCF [14]. Moreover, Dunet et al. [13] and Greulich et al. [8] classified splenic lesions and breast nodules as “major NCFs” whereas Mantini et al. [7] and our study classified those NCFs as “significant”. The differences in these studies generally result from the significance of differences in the classifications. We think that it is more appropriate to classify the NCFs in terms of follow-up or requiring treatment. Consistent with our results, Mantini et al. [7] reported the prevalence of major NCFs as 3.6%, and Roller et al. [15] reported the prevalence of major NCFs as 6.25% (25/400 patients).
The prevalence of previously unknown major NCFs in our study was 2% of the entire study population (8/400 patients) and is in accordance with recent studies [7,10,12,14]. A retrospective study by Mantini et al. [7], which included 500 patients, reported that 11 patients (2.2%) had previously undiagnosed major NCFs, and 35 (7%) patients had previously undiagnosed significant NCFs; they followed those patients for 15 months. However, this mentioned study did not include the frequency of significant and major NCF reports or what was reported in the previous official radiological reports [7]. Also, all major and significant NCFs were reported as the number of patients, which would mean that every patient had at least 1 major or significant NCF. However, in our study, we reported 25 previously undiagnosed major or significant NCFs in 22 patients. Moreover, Dunet et al. [14] reported that the prevalence of newly diagnosed major NCFs that changed patient management was between 1 and 2%, and Mantini et al. [7] reported the prevalence of newly diagnosed major NCFs as 1%.
To the best of our knowledge, our study is the first to compare significant and major NCF reporting frequency with the diagnoses reported on official radiology reports. Although we were able to detect an important number of significant and major NCFs on this retrospective review of MRI images, we discovered that some of the NCFs in our cohort were missed on official reports. However, no statistical difference was found between official MRI reports and second consensus reading for the detection of major NCFs. The reasons for unreported NCFs in cardiac MRI may vary. However, we know that radiologists are usually focused on cardiac pathologies during the review of cardiac MRI, which can be the cause for those missing significant and major NCF reports. Moreover, NCFs that are considered clinically unimportant during reporting may not have been reported.
There are some limitations to our study. The relatively small number of patients in our study and the retrospective design of the study are the most important limitations. However, the strong point of our study is that we did another review of all cardiac MRI images for NCF presence, as opposed to other studies, and we did not base our results just on previous official reports. Furthermore, we compared our systematic approach with the archived official cardiac MRI reports as it was used for reporting NCFs to estimate non-reported (missed) rates.
Conclusions
The detection of previously unknown NCFs during routine cardiac MRI examinations is frequent, the vast majority of identified NCFs are clinically insignificant, and the frequency of NCFs increases with age. Although a small number of previously unknown major NCFs have been identified on routine cardiac MRI, it has a significant impact on the diagnosis and treatment of patients. Although a minority of the previously unknown major NCFs were not reported in official MRI reports, no statistical difference was found between official MRI reports and second consensus readings for the detection of major NCFs.


