Introduction
The incidence of caesarean section (CS) has increased worldwide. The incidence of CS in Egypt (26%) and in Sudan (20%) is higher than the World Health Organization recommendation [1].
Routine catheterization before CS prevents iatrogenic urinary bladder injury and helps the postoperative assessment of urine output [2, 3]. On the other hand, prolonged catheterization may increase urine colonization and urinary tract infections (UTIs), which subsequently increases the healthcare system burden [4, 5].
Urinary tract infections are the commonest cause of antimicrobial therapy in hospitals, and about 80% of UTIs are catheter-associated [6]. The Infection Control Advisory Board agreed that there is strong evidence indicating that urinary catheterization should be avoided [7].
A systematic review concluded that routine urinary catheterization during CS is not necessary and could be harmful [8].
A Cochrane Review found that the evidence of using indwelling Foley’s catheters in women undergoing CS was insufficient [9].
The relationship between routine urinary catheterization during CS, and postoperative urinary symptoms and UTIs has not been intensively studied.
Therefore, this study was designed to detect the relationship between routine urinary catheterization and postoperative urinary symptoms and UTIs in women undergoing elective caesarean sections (ECSs).
Material and methods
One hundred women were included in this observational randomized study, which was conducted from August 2019 to August 2021 after departmental Ethical Committee approval (Obs_1508_19) and informed consent following the Declaration of Helsinki.
Inclusion criteria included women admitted for the following: 1) ECS for the first delivery due to abnormal foetal presentation (breech presentation or transverse lie); or 2) elective repeat CSs (ERCS) when they refused the trial of labour after previous CS [10, 11].
Women with a history of UTIs in current pregnancy, ≥ 2 previous CSs, medical disorders with pregnancy (anaemia, hypertension, diabetes mellitus, or preeclampsia), uterine overdistension (twin pregnancy, and/or polyhydramnios), delivered by emergency CSs (i.e. foetal distress and/or antepartum haemorrhage), women with premature rupture of membranes, surgical complications during previous CS, antibiotic therapy within the last 2 weeks, and women who refused to participate were excluded from this study.
The studied participants were informed that routine catheterization before CS prevents iatrogenic urinary bladder injury and helps the postoperative assessment of urine output [2, 3]. On the other hand, they were informed that routine urinary catheterization during CS is not necessary [8], and prolonged catheterization may increase UTIs (80% of UTIs are catheter-associated) [6]. Additionally, participants were informed that each participant who agreed and was included in the study had an equal chance to be in the catheterized (C) group or in the non-catheterized (NC) group according to the randomization table.
After the detailed explanation and obtaining the participants’ consents, the studied participants were randomly assigned using a computer-generated randomization table into 2 groups; the C group, including women who underwent ECS after insertion of an indwelling Foley’s catheter, and the NC group, including women who underwent ECSs without a Foley’s catheter (after self-voiding and evacuation of the bladder prior to the ECS).
Participants were thoroughly evaluated with abdominal and an ultrasound examinations for assessment of the foetal position, presentation, amniotic fluid volume, and placental site before the ECS.
The preoperative investigations were done according to the hospital’s protocol, including complete blood count, coagulation profile, liver, and kidney function tests.
All the ECSs were done after 38 weeks and before 38 weeks + 6 days. Participants received 4 doses of 6 mg dexamethasone intramuscularly 12 hours apart one week before the ECS to enhance the maturity of foetal lungs and to decrease the neonatal respiratory morbidity according to the hospital’s protocol [10], and Royal College of Obstetricians and Gynaecologists recommendations [12].
The Royal College of Obstetricians and Gynaecologists [12] and the Cochrane database [13] recommend a course of antenatal corticosteroids one week before the ECS done before 38 weeks + 6 days, after an informed discussion explaining the benefits and risks of antenatal corticosteroids.
Participants received a single dose of first-generation cephalosporin 30–60 min. before the ECS according to American College of Obstetricians and Gynecologists guidelines [14].
All ECSs were done under spinal anaesthesia. After a Joel-Cohen incision [15] and opening of the abdominal wall in layers, the visceral peritoneum was opened to create the bladder flap and to dissect the bladder away from the lower segment of the uterus [16, 17].
A transverse incision was done in the lower uterine segment, then the amniotic fluid was drained by suction, followed by delivery of the foetus and clamping of the umbilical cord. Oxytocin (20 IU, Novartis, Switzerland) over 500 m of 0.9% saline was given by IV infusion to manage the 3rd stage of labour actively [18, 19]. The uterine incision was repaired using No. 1 absorbable Vicryl sutures (Polyglycolic, Ethicon, USA) in 2 layers (after delivery of the placenta), followed by anterior abdominal wall closure in layers.
The Foley’s catheters were inserted for all participants in the C group in the operating theatre, under aseptic precautions according to the hospital protocol, after the anaesthetist’s approval, and before the ECS procedure. The Foley’s catheters were removed from all participants in the C group once they were free ambulant and had recovered from the effect of the spinal anaesthesia [20].
Urine samples (mid-stream) were collected from the studied participants using the first spontaneously voided urine for urine cultures, and antibiotics were only prescribed for participants with UTIs [21].
Participants were also asked about any abnormal postoperative urinary symptoms [dysuria (painful micturition), urgency (severe desire to urinate), frequency (> 7 voids during the daytime or > 2 voids at night-time), and/or urinary retention (inability to void 6 hours after removal of the urinary catheter with painful palpable bladder)] [22].
The presence of ≥ 105 of one bacterial species per millilitre of voided urine in women with UTI symptoms is diagnostic for UTIs. Women were instructed to place their legs apart, to clean the inner folds of the labia and urethral opening with water, then start to void and catch mid-stream urine for culture (to decrease the contamination of the specimen by introitus bacteria) [21, 22].
The participants’ data were analysed to detect the relationship between routine urinary catheterization and postoperative urinary symptoms and UTIs in women undergoing ECSs (main outcome).
Statistical analysis
G Power 3.1.9.7 was used for sample size calculation with 0.05 probability, 0.95% power, and 0.5 sample size [23–25], and the chi-square (χ2) test was used for statistical analysis.
Qualitative and quantitative data of the participants were analysed using the χ 2 test and Student’s t-test, respectively. The odds ratio (OR) of abnormal postoperative urinary symptoms and UTIs in relation to routine urinary catheterization in women undergoing ECSs was also calculated using MedCalc 20.106. P < 0.05 was considered significant.
Results
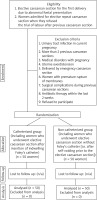
One hundred women were included in this observational randomized study for ECS due to abnormal foetal presentation or due to refused trial of labour after previous CS (Fig. 1).
Participants were randomly assigned into the C group, including women who underwent ECS after insertion of an indwelling Foley’s catheter, and the NC group, including women who underwent ECS without a Foley’s catheter (after self-voiding and evacuation of the bladder prior to the ECS) (Fig. 1).
The Foley’s catheters were inserted for all participants in the C group in the operating theatre, under aseptic precautions, after the anaesthetist’s approval, and before the ECS procedure. The Foley’s catheters were removed from all participants in the C group once they were free ambulant and had recovered from the effect of the spinal anaesthesia [20].
Mid-stream urine samples were collected from the studied participants using the first spontaneously voided urine for urine cultures, and antibiotics were only prescribed for participants with UTIs.
Participants were also asked about any abnormal postoperative urinary symptoms (dysuria, frequency, urgency, and/or urinary retention).
The participants’ data were analysed to detect the relationship between routine urinary catheterization and postoperative urinary symptoms and UTIs in women undergoing ECS.
There was no significant difference between the C group and the NC group regarding the maternal age (25.56 ±3.8 years vs. 26.1 ±3.3, respectively) (p = 0.1), body mass index (BMI) (28.1 ±2.4 kg/m2 vs. 27.4 ±2.1, respectively) (p = 0.2), preoperative haemoglobin (12.2 ±0.6 gms% vs. 12.1 ±0.5, respectively) (p = 0.1), and duration of the ECS (44.9 ±3.3 min. vs. 45.5 ± 3.8, respectively) (p = 0.8) (Table 1).
Table 1
Participants’ characteristics, postoperative urinary symptoms, urinary tract infections, and postoperative hospital stays
The postoperative urinary symptoms
The postoperative dysuria, frequency, and urgency were significantly higher in the C group compared to the NC group [36% (18/50), 40% (20/50), and 34% (17/50) vs. 8% (4/50), 6% (3/50), and 6% (3/50), respectively] (p = 0.006, 0.001 and 0.004; respectively) (Table 1).
The routine catheterization in women undergoing ECSs increases the odds of post-operative dysuria [OR 6.5 (95% CI: 2.0–20.9); p = 0.001], urinary frequency [OR 10.4 (95% CI: 2.9–38.2); p = 0.0004], and urgency [OR 8.1 (95% CI: 2.2–29.8); p = 0.001] (Table 2).
Table 2
The odds of urinary symptoms and urinary tract infections in relation to routine urinary catheterization in women undergoing elective caesarean section
Urinary tract infections and postoperative antimicrobials
The number of urinary tract infections and postoperative antimicrobials used were significantly higher in the C group compared to the NC group [40% (20/50) and 40% (20/50) vs. 6% (3/50) and 6% (3/50), respectively] (p = 0.001 and 0.001, respectively) (Table 1).
The routine catheterization in women undergoing ECSs increases the odds of UTIs [OR 10.4 (95% CI: 2.9–38.2); p = 0.0004] and the number of post-operative antimicrobials used [OR 10.4 (95% CI: 2.9–38.2); p = 0.0004] (Table 2).
Postoperative hospital stay after elective caesarean section
The post-operative hospital-stay after the ECS was significantly higher in the C group compared to the NC group (5.4 ±1.8 days vs. 3.8 ±1.15, respectively) (p = 0.001) (Table 1).
Discussion
The Infection Control Advisory Board agreed that there is good evidence that urinary catheterization should be avoided [7].
A Cochrane Review found evidence that using indwelling Foley’s catheters in women undergoing CSs was insufficient [9]. The relation between routine urinary catheterization during CSs, and postoperative urinary symptoms and UTIs not intensively studied. Therefore, this study was designed to detect the relationship between routine urinary catheterization and postoperative urinary symptoms and UTIs in women undergoing ECS.
One hundred women were included in this observational study and randomized into the C group, including women who underwent ECSs after insertion of an indwelling Foley’s catheter and the NC group, including women who underwent ECSs without a Foley’s catheter (after self-voiding prior to the ECS).
The Foley’s catheters were inserted for all participants in the C group in the operating theatre, under aseptic precautions, after the anaesthetist’s approval, and before the ECS procedure. The Foley’s catheters were removed from all participants in the C group once they were free ambulant and had recovered from the effect of the spinal anaesthesia [20].
Mid-stream urine samples were collected from the studied participants using the first spontaneously voided urine for urine cultures, and antibiotics were only prescribed for participants with UTIs.
Participants were also asked about any abnormal postoperative urinary symptoms (dysuria, frequency, urgency, and/or urinary retention).
The participants’ data were analysed to detect the relationship between routine urinary catheterization and postoperative urinary symptoms and UTIs in women undergoing ECS.
The postoperative urinary symptoms including dysuria, frequency, and urgency were significantly higher in the C group compared to the NC group [36% (18/50), 40% (20/50), and 34% (17/50), vs. 8% (4/50), 6% (3/50), and 6% (3/50), respectively], (p = 0.006, 0.001, and 0.004, respectively).
Additionally, the routine catheterization in women undergoing ECS increases the odds of post-operative dysuria [OR 6.5 (95% CI: 2.0–20.9); p = 0.001], urinary frequency [OR 10.4 (95% CI: 2.9–38.2); p = 0.0004], and urgency [OR 8.1 (95% CI: 2.2–29.8); p = 0.001].
The numbers of urinary tract infections and postoperative antimicrobials were significantly higher in the C group compared to the NC group [40% (20/50) and 40% (20/50) vs. 6% (3/50) and 6% (3/50), respectively] (p = 0.001 and 0.001, respectively).
Additionally, routine catheterization in women undergoing ECSs increases the odds of UTIs [OR 10.4 (95% CI: 2.9–38.2); p = 0.0004] and the number of post-operative antimicrobials used [OR 10.4 (95% CI: 2.9–38.2); p = 0.0004].
The postoperative hospital stay after the ECS was also significantly longer in the C group compared to the NC group (5.4 ±1.8 days vs. 3.8 ±1.15 days, respectively) (p = 0.001).
Urinary tract infections are the commonest cause of antimicrobial therapy in hospitals, and about 80% of UTIs are catheter-associated [6].
The Infection Control Advisory Board agreed that there is good evidence that urinary catheterization should be avoided [7].
A randomized controlled trial (RCT) found that the routine urinary catheterization in minor gynaecological surgeries increases the risk of asymptomatic bacteriuria and UTIs [6].
Pandey et al. [26] found that NC patients during CS had significantly earlier ambulation, shorter hospital stay, less voiding discomfort and postoperative antibiotics, and low UTI incidence.
A multicentre RCT concluded that CS without an indwelling urinary catheter was more convenient, with increased intraoperative complications or postoperative urinary retention, while indwelling urinary catheterization during CSs in haemodynamically stable patients was not beneficial [27].
Another RCT concluded that CS can be done safely without urethral catheterization with reduced postoperative morbidities [28].
A systematic review concluded that the routine use of indwelling Foley’s catheters during CS in haemodynamically stable women is not necessary [8].
Ghoreishi [3] also found that routine use of urinary catheters during CS in haemodynamically stable women was not necessary.
This study found that postoperative dysuria, frequency, and urgency were significantly higher in the C group compared to the NC group (36%, 40%, and 34% vs. 8%, 6%, and 6%, respectively).
The numbers of urinary tract infections and used postoperative antimicrobials were significantly higher in the C group compared to the NC group (40% and 40% vs. 6% and 6%, respectively).
This study was the first conducted in our region, and it concluded that routine urinary catheterizations in women undergoing ECS significantly increase the odds of postoperative dysuria, frequency, urgency, UTIs, and the number of postoperative antimicrobials used.
No limitations were faced during this study. Further studies are needed to evaluate whether routine urinary catheterization in women undergoing CS is still necessary.
Conclusions
Routine urinary catheterizations in women undergoing ECS significantly increase the odds of postoperative dysuria, frequency, urgency, UTIs, and the number of postoperative antimicrobials used. Further studies are needed to evaluate whether routine urinary catheterization in women undergoing CS is still necessary.