Introduction
The prolactin (PRL) has a variety of biological functions in addition to its role in lactation, including development of myelin sheath of the central nervous system neuronal axons and lung surfactant [1–3].
The secretion of PRL from the lactotrophs of the anterior pituitary gland is regulated by different regulatory mechanisms [4] and by internal and external factors [5].
Normally, the PRL secretion is under the inhibitory control of dopamine [5]. Different peptides and growth factors can stimulate the PRL secretion either through its inhibitory action on dopamine or through direct effect on the PRL secreting lactotrophs [5].
Lactation, stress and steroid hormones such as the ovarian estrogen increase the PRL production [6].
The thyroid releasing hormone (TRH) increases the thyroid stimulating hormone (TSH) secretion which subsequently increases the PRL secretion [7].
Vitamin D (Vit. D) deficiency was reported with Hashimoto’s thyroiditis (HT) [8, 9]. Appunni et al. recently found 25.6% of their studied participants with hypothyroidism had deficient Vit. D compared to 20.6% of healthy controls [10]. Appunni et al. also reported increased odds of hypothyroidism among Vit. D deficient participants [10].
The relation between Vit. D and PRL are conflicting in the previous studies. Although, Saki et al. found the PRL was more potent in increasing the serum 1.25(OH)2D than estrogen in animal model [11], and Törnquist et al. found the PRL secretion increased from the animal cells incubated with 1.25(OH)2D [12].
Haug et al. found the treatment of animal cells with 1.25(OH)2D caused an inhibition on PRL secretion [13]. The 25(OH)D is the most extensively used biomarker to measure the serum Vit. D [14]. Therefore, this cross-section research designed to detect whether there is a relation between Vit. D and adolescents’ serum PRL or not.
Material and methods
Hundred and seventy-six (176) school attendant adolescent girls were recruited for this cross-sectional research which was conducted in West Kazakhstan (Aktobe) over two years (2021–2022) to detect whether there is a relation between Vit. D and adolescents’ serum PRL or not.
The adolescents were recruited for the current research, after the ethics committee of West Kazakhstan Medical University (WKMU) approval and after informed consent from the adolescents and their guardians.
After thorough evaluation, the weight and height of the studied adolescents were measured to calculate the body mass index (BMI) [detected from the adolescents’ weight divided by meters-squared height (kg/m2)] [15, 16].
Inclusion criteria include adolescents (12–18 years-old), with regular menstrual cycles, normal BMI (18.5–24.9), without any known chronic or endocrine disorders.
Exclusion criteria include adolescents < 12 years-old or >18 years-old, underweight (BMI ≤ 18.5), overweight (BMI 25–29.9) or obese (BMI > 30) [15, 17], with irregular menstrual cycles, known medical disorders (i.e., diabetes, or hypertension), known endocrine disorders (i.e., thyroid, or hyperprolactinemia), and received exogenous hormones within the last year.
Regular menstrual cycles are defined as menstrual flow on regular basis every 21–35 days. Diabetes defined according to the American Diabetic Association as metabolic disorders due to defective insulin secretion or action. Diabetes diagnosed when the glycosylated hemoglobin (HbA1C) is ≥ 6.5% and fasting plasma sugar is ≥ 126 mg/dl or 2-hrs. plasma sugar is ≥ 200 mg/dl [18].
Hypertension diagnosed when the blood pressure is ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic (on two separate sessions) [19].
Blood samples were taken from adolescents to measure TSH (normal 0.4–4.0 mIU/ml) [20], free thyroxine [T4 (normal 0.9–2.3 ng/dl)], PRL (normal < 29 ng/ml) [21], HbA1C (normal < 6.5%) [14] and 25(OH)D. The clinical hypothyroidism was diagnosed with high TSH and low free T4 [20].
The adolescents` blood was centrifugated and stored at –20°C for the quantitative 25(OH)D evaluation. The 25(OH)D level was measured using the Architect (Abbott, Longford, Ireland). The Architect 25(OH)D evaluation is a delayed 1-step chemiluminescence microparticles evaluation uses the Architect 25(OH)D Reagent Kit. The microparticles contains a sheep anti-25(OH)D antibody, and a biotinylated 25(OH)D anti-biotin antibodies-labelled conjugate complex [22].
Vitamin D deficiency was diagnosed when the serum 25(OH)D was > 20 ng/ml, while serum 25(OH)D > 30 ng/ml was considered as normal serum Vit. D [22].
The studied adolescent girls were classified into study group [25(OH)D deficient (88 adolescents)] and controls [normal 25(OH)D (88 adolescents)]. The acquired adolescents’ variables were analyzed to detect whether there is a relation between Vit. D and adolescents’ serum PRL or not.
Statistical analysis
The G Power 3.1.9.7 with 0.05 probability, 0.95% power, 0.5 sample size and Student t-test for statistical analysis was used for sample size calculation [23, 24]. The variables of the studied adolescent girls were checked for normal distribution using the Shapiro-Francia test [25] before statistical analysis. The acquired adolescents’ variables were analyzed using the Student t-test and Pearson’s correlation. p < 0.05 considered significant.
Ethical considerations
Adolescents included in this research, after the ethics committee of WKMU approval (No. 10–04 October 2020) and after informed consent from the adolescents and their guardians.
Grant funding for scientific and/or technical projects for the years 2021–2022- KZ- IRN AP09563004 – Supervisor (AA).
Results
Hundred and seventy-six school attendant adolescent girls between 12–18 years-old were recruited for this cross-sectional research to detect whether there is a relation between Vit. D and adolescents’ serum PRL or not.
The studied adolescents were classified into study group [25(OH)D deficient (88 adolescents)] and controls [normal 25(OH)D (88 adolescents)].
The was no difference between the study group and controls regarding the mean age (15.9 ±1.27 years in the study group vs. 15.99 ±1.1 for the controls), (p = 0.09).
About 85.2% (75/88) and 14.8% (13/88) of the studied adolescents in the study group were high-school and mid-school attendants, respectively compared to 86.4% (76/88) and 13.6% (12/88) of the controls (p = 1.0 and 0.85, respectively). About 76.1% (67/88) and 21.6% (19/88) of the studied adolescents in the study group have average and above average family income, respectively compared to 71.6% (63/88) and 23.9% (21/88) of the controls (p = 0.79 and 0.77, respectively), and 2.3% (2/88) of the studied adolescents in the study group have below average family income compared to 4.5% (4/88) of the controls (p = 0.4) (Table 1). The average family income/month in the Republic of Kazakhstan is 770 US dollars/month [26].
Table 1
The studied adolescents` socioeconomic characteristics, weight, height, body mass index, 25(OH)D, prolactin, and thyroid profile
There was no difference between the study group and controls regarding the mean weight (58.5 ±2.1 kg vs. 58.0 ±2.9, respectively), (p = 0.9), height (157.5 ±1.6 cm vs. 157.7 ±2.3, respectively), (p = 0.9) and BMI (23.5 ±0.7 kg/m2 vs. 23.26 ±0.96, respectively), (p = 0.9).
The 25(OH)D was statistically lower in the study group than normal controls (13.8 ±2.5 ng/ml vs. 35.6 ±2.02, respectively), [p = 0.02 (95% CI: –22.5, –21.8, –21.1)] (Table 1).
The serum TSH and PRL were statistically higher in the study group than normal controls (3.73 ±1.45 mIU/ml and 47.5 ±7.6 ng/ml vs. 2.67 ±1.0 and 10.8 ±5.1, respectively), (p = 0.0003, and p = 0.0001, respectively). The free T4 was statistically lower in the study group than normal controls (1.4 ±0.6 ng/ml vs. 1.5 ±0.4), [p = 0.0001 (95% CI: –0.25, –0.1, 0.05)] (Table 1).
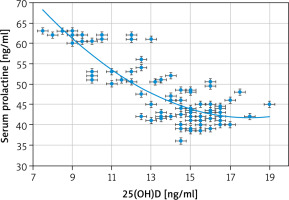
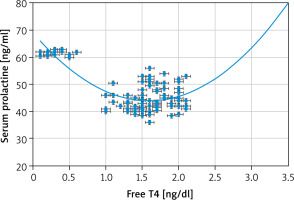
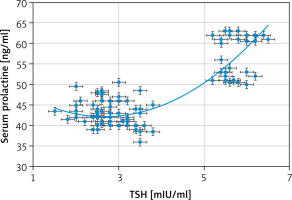
Strong negative associations between the serum PRL and 25(OH)D [r = –0.803 (p < 0.00001)] (Fig. 1) and between the serum PRL and free T4 [r = –0.6959 (p < 0.00001)] (Fig. 2) were detected in this study. Additionally, there was a strong positive association between the serum PRL and TSH [r = 0.8137 (p < 0.00001)] (Fig. 3).
Discussion
The relation between Vit. D and PRL are conflicting in the previous studies. Although, Saki et al. found the PRL was more potent in increasing the serum 1.25(OH)2D than estrogen in animal model [11], and Törnquist et al. found the PRL secretion increased from the animal cells incubated with 1.25(OH)2D [12].
Haug et al. found the treatment of animal cells with 1.25(OH)2D caused an inhibition on PRL secretion [13]. Therefore, hundred and seventy-six (176) school attendant adolescent girls between 12–18 years-old were recruited for this cross-sectional research to detect whether there is a relation between Vit. D and adolescents’ serum PRL or not. The studied adolescents were classified into study group [25(OH)D deficient (88 adolescents)] and controls [normal 25(OH)D (88 adolescents)].
The 25(OH)D is the most extensively used biomarker to measure the serum Vit. D [14]. It has a long half-life [14], and it reflects the cutaneous production and the intake of Vit. D [27].
The 1.25(OH)2D level is not ideal indicator for the Vit. D status because it is only decreased after severe Vit. D deficiency [27]. Additionally, it has a short half-life, and it is regulated by the PTH, calcium and phosphate [27].
The 25(OH)D relation to thyroid and prolactin disorders
Although, Saki et al. found the PRL was more potent in increasing the serum 1.25(OH)2D than estrogen in animal model [11] and Törnquist et al. found the PRL secretion increased from the animal cells incubated with 1.25(OH)2D [12].
The serum TSH and PRL in this study were statistically higher in the study group than normal controls (3.73 ±1.45 mIU/ml and 47.5 ±7.6 ng/ml vs. 2.67 ±1.0 and 10.8 ±5.1, respectively), (p = 0.0003, and p = 0.0001, respectively). A strong negative association between the serum PRL and 25(OH)D [r = –0.803 (p < 0.00001)] and a strong positive association between the serum PRL and TSH [r = 0.8137 (p < 0.00001)] were detected in this study.
Vitamin D deficiency was reported with hypothyroidism especially HT [8, 9]. Chao et al. found the TSH was significantly higher in 25(OH)D deficient participants than controls [28]. Bozkurt et al. found patients with hypothyroidism irrespective the hypothyroidism was due to autoimmune thyroiditis (AITDs) or not had a low Vit. D [29]. Kim et al. found the low Vit. D was independently associated with HT and high TSH [30]. Fang et al. confirmed a positive association between the antithyroid antibodies and Vit. D deficiency [31].
Moreover, Mackawy et al. reported a strong negative relation between Vit. D and TSH [32]. Recently, Appunni et al. analyzed 7943 participants and found that 25.6% of their studied participants with hypothyroid had deficient Vit. D compared to 20.6% of healthy controls [10]. Appunni et al. also reported increased odds of hypothyroidism among Vit. D deficient participants [10].
The hypothyroidism is associated with elevated TSH through the feedback mechanism on the thyrotropes of the anterior pituitary [33]. The thyroid stimulating hormone stimulates the anterior pituitary lactotrophs with subsequent increased PRL secretion [7].
Haug et al. found the treatment of animal cells with 1.25(OH)2D caused an inhibition on PRL secretion [13]. Additionally, Haug et al. found the 1.25(OH)2D inhibits the TRH and estrogens stimulation on PRL produ- ction [13].
Krysiak et al. [34] and Aboelnaga et al. [35] found that Vit. D administered to patients with prolactinoma increases the 25(OH)D and reduces the serum PRL [34].
The Haug et al. [13], and Krysiak et al. [34] findings support the strong negative association between the serum PRL, and 25(OH)D found in this study.
The association between Vit. D and serum PRL and the effect of Vit. D intake on the serum PRL need to be confirmed in further studies.
This research was the first cross-sectional research conducted in West Kazakhstan (Aktobe) to detect the relation between Vit. D and adolescents’ serum PRL. The serum TSH and PRL were statistically higher in the studied adolescents than normal controls. A strong negative association between the serum PRL and 25(OH)D and a strong positive association between the serum PRL and TSH were detected in this study. Failure to detect the causes of hyperprolactinemia in the studied adolescents and the effect of Vit. D intake on the serum PRL were the limitations of this study.
Conclusions
A strong negative association between the serum PRL and 25(OH)D and a strong positive association between the serum PRL and TSH were detected in this study. This study recommends further studies to confirm the relation between Vit. D and PRL and screening Vit. D deficient adolescents for PRL and thyroid disorders.