Introduction
Endometriosis is a common benign and chronic inflammatory gynaecological disease due to functional endometrial glands and stroma in an ectopic location outside the uterine cavity [1]. It affects 5-10% of reproductive age group women, with a peak age of 24-29 years [2,3]. However, women with infertility and chronic pelvic pain have an even greater prevalence, accounting for 30-50% and 90% cases, respectively [4,5]. Laparoscopy followed by histopathological confirmation is the gold standard for diagnosis but is an invasive procedure. Magnetic resonance imaging (MRI) is an excellent non-invasive modality that helps in non-invasive diagnosis, with excellent delineation of the disease extent, and thus provides a presurgical mapping of the disease, which is helpful for the operating surgeon [6].
Aetiology and pathogenesis
The exact pathogenesis is still under research and is likely to be multifactorial; however, several theories have been suggested [7]. The most widely accepted theory is Sampson’s implantation or metastatic theory, which suggests retrograde menstruation to be responsible for deposits in the pelvic cavity [8,9]. The theory is supported by the fact that implants are present in the dependent portion of the pelvic cavity. It also explains the increased incidence of endometriosis in patients with Mullerian anomalies, which obstruct menstrual flow and thus retrograde menstruation [10]. The drawback of the theory is that a more significant proportion of patients experience retrograde menstruation, but endometriosis affects only 10% of women [11]. This drawback is explained by immunological theory, which states that normally refluxed menstrual endometrium is cleared from the peritoneal cavity by macrophages, natural killer cells, and lymphocytes preventing endometriosis in most cases. However, there is reduced immunological clearance of refluxed endometrium from the peritoneal cavity, causing endometriosis in some women with immune system dysfunction.
Many authors have also described other theories, such as metaplastic theory and induction theory. Metaplastic theory suggests the differentiation of serosal surfaces or celomic epithelium into endometrial tissue, and arguments given in favour include the fact that endometriosis can also occur in patients with uterine agenesis, gonadal dysgenesis, and Turner syndrome [12]. Induction theory combines Sampson’s theory and metaplastic theory and discusses the secretion of substances by the ectopic endometrial tissues, which induce differentiation of the serosal surfaces [12]. Metastatic theory or Halban’s theory also explains the presence of endometriosis at distant and unusual sites like skin, umbilicus, bowel wall, pleura lungs, ureter, endocardium, pelvic lymph nodes, and retroperitoneum and extremely rare sites such as brain.
Stem cell theory is the most recent theory that states endometrial progenitor cells shed during menstruation spread to the peritoneum via retrograde menstruation [13-15]. Furthermore, endometriosis beginning with endometrial stem cells is more severe than from more differentiated cells [11].
These ectopic implants are hormonally responsive and undergo cyclical changes like the endometrium, but it accumulates blood and blood products without a passage for drainage. After looking at the various theories, the logical risk factors would be those that increase the exposure to menstruation, such as early age of menarche, short menstrual cycle, heavy menstrual flow, nulliparity, and Mullerian anomalies [16].
Classification of endometriosis
Endometriosis has 3 subtypes: endometriomas, superficial, and deep infiltrating endometriosis (DIE), which can be pelvic and extrapelvic in location [17]. Endometrioma is a chronic retention cyst arising from endometrial deposits within the ovaries and represents the most common form of endometriosis. Superficial endometriosis (Sampson’s disease, non-invasive implants) refers to endometrial implants on the surface of pelvic organs or less than 5 mm deep into the peritoneum. These implants are below the resolution of current imaging modalities and are not visible on imaging, and are diagnosed on diagnostic laparoscopy. These superficial implants appear as either red, white, or bluish/black lesions on laparoscopy, depending on the disease activity. Red lesions are active and highly vascular, whereas white lesions represent later phases of red lesions with mixed inflammatory and fibrotic phases. Black or bluish-black lesions, also referred to as “powder burns”, represent cystic degeneration, scarring, and hemosiderin deposition [11]. DIE, also called solid infiltrating type, refers to endometrial implants greater than 5 mm deep into the tissue or peritoneum, and is a significant cause of pelvic pain and infertility. Most of the endometriotic lesions visible on MRI represent DIE, and the concomitant presence of endometrioma is considered a marker for severe DIE and 4-5 times increased risk of associated intestinal, vaginal, and ureteral lesions [18].
Clinical presentation
Endometriosis can be asymptomatic; however, chronic pelvic pain, infertility, and dyspareunia are the most common complaints and constitute a characteristic triad of endo-metriosis. Cyclical pain coinciding with menstruation is the expected presentation, but the clinical presentation can be varied and vague and depends on the location and organ involvement [19]. The most frequent anatomical locations in order of descending frequency are as follows: ovaries (endometrioma), pelvic peritoneum including pouch of Douglas, vesicouterine pouch, and rectovaginal septum, deep lesions of pelvic subperitoneal space, gastrointestinal system, and urinary system [20]. Uterosacral ligaments are the most common site of DIE and the second most common site after endometrioma [20]. The rectosigmoid junction is the most common intestinal segment affected [21]. Deep dyspareunia is primarily associated with rectovaginal and vaginal endometriosis, while dyschezia is related to lesions in the rectouterine pouch [22]. Bladder involvement presents with dysuria rather than cyclical haematuria, and abdominal wall involvement may be associated with constant pain [23].
Diagnosis of endometriosis
It is still clinically challenging to diagnose endometriosis because the symptoms are varied and non-specific. However, there are certain clinical clues, including fixed retroverted uterus on bimanual vaginal examination. There may be tenderness and nodularity (cobblestone feel) in the pouch of Douglas and on the uterosacral ligament as well as adnexal mass due to endometrioma [24]. Laparoscopy followed by histopathological examination is the gold standard for diagnosis, but it is invasive [25].
Ultrasonography, including transvaginal sonography (TVS) and transrectal sonography (TRS), is the first-line imaging modality. On ultrasonography, endometrioma appears to be a well-defined thick-walled cystic lesion with uniform ground-glass internal echoes and no internal vascularity (Figure 1). Endometriotic implants larger than 1.5 cm are also visible on ultrasound [12]. TRS is highly valuable in the evaluation of implants in the rectosigmoid and rectovaginal septum. Endometriotic implants appear as subtle hypoechoic nodular or infiltrating regions in the classic locations in the pelvis. Simple ultrasound manoeuvre of sliding sign can evaluate subtle adhesions in the pelvis by demonstrating relative mobility between the adjacent organs [26]. Ultrasound can also be helpful in the evaluation of endometriotic implants in superficial locations such as in the abdominal wall (Figure 2). The drawbacks of the ultrasound are decreased sensitivity in the detection of endometriosis in the anterior compartment (bladder and vesicouterine pouch) and middle compartment (torus uterinus and round ligaments), and, of course, the operator dependence [26].
Figure 1
Ultrasonographic appearance of endometrioma. Transvaginal scan shows a large well-defined unilocular cystic lesion in left adnexa adjacent to the uterus (Ut) with homogenous low-level internal echoes within giving “ground glass appearance”. The wall of the lesion is smooth with no evidence of septations or solid components within
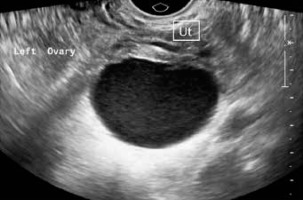
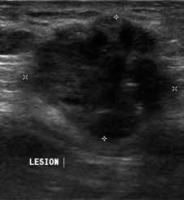
Figure 2
Sonographic appearance of scar endometriosis. Ultrasound image shows an ill-defined heterogeneously hypoechoic lesion with irregular margins in the infraumbilical region in the anterior midline
Computed tomography (CT) is not the preferred initial modality to evaluate endometriosis; however, endometriosis can be detected incidentally on the scan acquired for unrelated reasons, especially in unusual locations (Figure 3). CT is also done for evaluation of endometriosis involving the gastrointestinal and genitourinary systems [27].
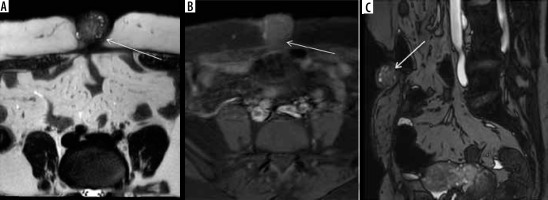
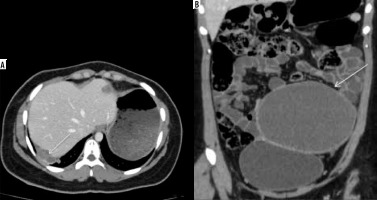
Figure 3
Diaphragmatic endometriosis. A) Contrast-enhanced computed tomography (CECT) axial image in venous phase demonstrate small cystic lesion with enhancing wall along right hemidiaphragm (white arrow). B) CECT coronal image of the same patient depicts a large well defined abdominopelvic cystic lesion (white arrow), which was operated and confirmed to be endometrioma on histopathological examination
MRI is an excellent non-invasive modality with superior contrast resolution that helps in non-invasive diagnosis and excellent delineation of the disease extent, and thus provides a presurgical mapping of the disease, which is helpful for the operating surgeon [28]. Therefore, the rest of the article is focused on MRI imaging in endometriosis.
Magnetic resonance imaging technique and protocol
In 2019, the European Society of Urogenital Radiology (ESUR) published the updated guidelines for female pelvic imaging in various scenarios in a booklet called “ESUR Quick Guide to Female Pelvis Imaging”. According to these guidelines, MRI for endometriosis evaluation can be performed on either a 1.5- or 3-Tesla MRI with a pelvic phased-array coil recommended. Patients should be fasting 3-6 hours before the examination. The use of bowel preparation and antiperistaltic agents are recommended to reduce motion artifacts. The bladder should be moderately distended because if overdistended, it will cause detrusor contraction and motion artifacts. Vaginal and rectal opacification using gel is optional to improve diagnostic accuracy. The protocol should include at least two 2D T2-weighted sequences in orthogonal planes (at least axial and sagittal) and a T1-weighted sequence with and without fat suppression (Figure 4). Diffusion-weighted and dynamic contrastenhanced sequences are not recommended except in cases of indeterminate adnexal mass or suspected malignancy [29].
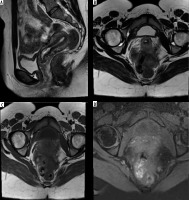
Figure 4
Recommended magnetic resonance imaging (MRI) sequences for endometriosis. According to the European Society of Urogenital Radiology (ESUR) guidelines, MRI protocol should include at least two 2D T2-weighted sequences at least sagittal (A) and axial (B) and a T1-weighted sequence without (C) and with fat suppression (D)
Furthermore, in suspected malignancy, subtraction imaging should be performed to assess for enhancement in solid nodules. Susceptibility-weighted imaging for endometriosis is currently under evaluation. We acquired all the scans as per the ESUR guidelines on a 3.0-T Phillips scanner with 3-4 hours of fasting, and intravenous Buscopan (Scopolamine-N-butyl bromide) was administered at the time of scanning to reduce bowel peristalsis, uterine contractions, and motion artifacts. Although not recommended, the contrast was also given to evaluate endometrial deposits wherever required [29].
Magnetic resonance imaging anatomy and interpretation
In 2019 the ESUR published consensus guidelines for MRI of pelvic endometriosis, which described the MRI techniques to be followed and gave suggestions on the reporting of findings. It was suggested that the pelvic compartment approach be used to describe the imaging findings, and the inclusion of assessing the bowel and urinary system [29]. Coutinho et al. first used this compartment approach in the evaluation of deep infiltrative endometriosis [30]. They divided the pelvis into 3 compartments: anterior, middle, and posterior (Figure 5). The anterior compartment is the space between the posterior surface of the pubic symphysis and the anterior surface of the uterus. It includes prevesical space, round ligaments, urinary bladder, distal ureters, vesicouterine space, and vesicovaginal space. Uterus, ovaries, fallopian tubes, broad ligament, and vagina fall under the middle compartment. The posterior compartment, which is the most frequent location of DIE, includes the rectosigmoid colon, rectouterine space, rectovaginal space, and uterosacral ligaments (Figure 6) [28,30]. After localizing the lesion to one specific compartment, it should be further described in terms of size, morphology, signal pattern, adhesions, and associated anatomical distortion. Therefore, this article will discuss the imaging findings of the various endometriotic lesions according to the compartment approach, followed by a brief mention of rare extrapelvic sites such as gastrointestinal and abdominal wall endometriosis.
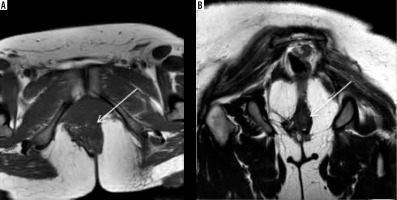
Figure 5
Anatomy of the female pelvis on sagittal section. A) Sketch diagram of the sagittal section of female magnetic resonance pelvis showing normal anatomy. Figure B is the corresponding sagittal T2W image demonstrating the anatomic structures that are commonly affected in endometriosis namely prevesical space (orange), peritoneal refection (green), vesicovaginal septum (light blue), vesicouterine space (pink), rectouterine space (dark blue), rectovaginal septum (violet), and presacral space (red). Figure C depicts the 3 anatomic compartments, namely, anterior (bounded by yellow line), middle (bounded by pink line), and posterior (bounded by orange line), used in reporting deep infiltrative endometriosis
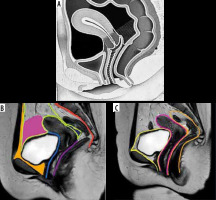
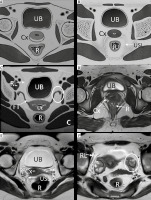
Figure 6
Anatomy of female pelvis on axial sections: Sketch diagrams (A, B, C) and corresponding T2W axial images (D, E, F) demonstrate axial anatomy of the pelvis at the inferior level of cervix (A, D), uterocervical junction in the middle (level of torus uterinus and uterosacral ligaments in B and E) and superiorly at the level of fallopian tubes and ovaries (C, F), respectively. Round ligaments are also seen at this superior level. UB – urinary bladder, Cx – cervix, R – rectum, USL – uterosacral ligament, Ut – uterus, FT – fallopian tube, RL – round ligament, and ovaries (white circles)
Magnetic resonance imaging findings in endometriosis
Endometrioma
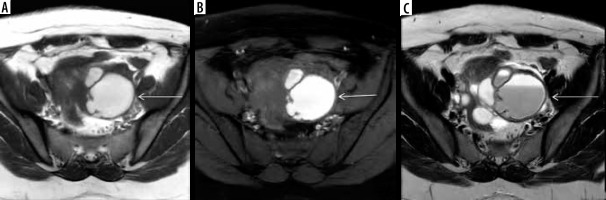
Endometrioma, also known as chocolate cyst, is an often large, frequently bilateral, well-defined, thick-walled multilocular cystic lesion. It shows uniform hyperintensity on the T1W sequence with no signal suppression on the T1W fat-suppressed sequence (Figure 7A, B). This hyperintensity is described as “light bulb bright” and occurs due to the high concentrations of methemoglobin and proteins resulting from repeated haemorrhage [31]. In 1992, Togashi et al. described a phenomenon called “T2 shading”, referring to the focal or uniform loss of signal on the T2W sequence (Figure 7C) [32]. They found sensitivity, specificity, and accuracy of T2 shading sign for the differentiation of endometrioma from other adnexal lesions to be 90%, 98%, and 96%, respectively [32]. This loss of the T2 signal is also because of high concentrations of methemoglobin and proteins resulting from repeated haemorrhage. T2 signal is usually hypointense, but it can be variable depending on the age of blood products. Due to chronic repetitive haemorrhage, other findings on T2w include a dark fibrotic rim from hemosiderin deposition and T2 dark spots. Corwin et al. proposed a new sign called the “T2 dark spot sign”, which appears as discrete markedly hypointense foci in the periphery of the cyst but separate from the wall due to chronic haemorrhage [33]. This sign is a more specific sign of endometriosis occurring due to high iron and protein concentrations, with one study having sensitivity, specificity, and positive predictive values of 36%, 93%, and 89%, respectively, differentiating endometriosis from the haemorrhagic cyst [33]. Endometrioma shows low ADC values on diffusion-weighted sequences partly because of restricted diffusion owing to thick and viscous contents and partly due to T2 blackout effects [27,31]. On contrast images, it shows peripheral enhancement, unlike haemorrhagic cyst.
Figure 7
Endometrioma: T1W axial image (A) of the pelvis showing well-defined, uniformly hyperintense cyst (white arrows) with no suppression of signal on T1 fat suppressed sequence (B), rather becoming more hyperintense giving a “light bulb appearance”. The lesion shows loss of the signal on T2W images (C) suggesting “T2 shading” and peripheral hypointense wall
Differential diagnoses
Two primary differential diagnoses include haemorrhagic cyst and mature ovarian teratoma.
Differentiating features of endometrioma and haemorrhagic cyst are summarised in Table 1.
Table 1
Differentiating magnetic resonance imaging features between endometrioma and haemorrhagic cyst
Mature cystic teratoma can be differentiated from endometrioma on T1W fat-suppressed images because teratoma shows signal suppression while endometrioma does not; instead, it becomes more conspicuous (Figure 7B). However, loss of T1W hyperintensity on STIR is not specific to fat; endometrioma and haemorrhagic cyst may mimic mature cystic teratoma on STIR imaging because they have similar relaxation times as fat. Therefore, sequences with chemical suppression of fat should be used to evaluate endometrioma to avoid this potential pitfall [29].
Complications of endometrioma
Malignant transformation: Around 1-2% of women undergo a malignant transformation for unknown reasons, and this occurs 10-20 years earlier than those without endometriosis [27]. Fortunately, endometriosis-associated malignancy tends to be low grade and has a good prognosis. Endometrioid carcinoma (66.7%) and clear cell carcinoma (14.8%) are the most common histological subtypes associated with endometriosis [34]. In addition, postmenopausal status and endometrioma > 9 cm are independent predictors of malignant transformation [35]. On MRI, the most specific feature suggestive of malignant transformation is solid enhancing mural nodules, best visualized on subtraction imaging because of the intrinsic T1W hyperintensity of endometrioma. Other features suggesting malignant transformation are a sudden increase in the size of the cyst, absence of T2 shading, nodule size > 3 cm, ascites, and peritoneal implants [34].
Decidualization: With increased progesterone levels in pregnancy, endometrioma undergoes decidual changes in the endometrial stromal tissue, manifested by vascular mural nodules, and thus mimicking ovarian malignancy. MRI features helpful in their diagnosis are their signal intensity, which is similar to decidualized endometrium. They are managed conservatively and usually resolve or regress after childbirth [31].
Ovarian torsion and rupture: Because of the association of endometrioma with DIE, there are usually dense adhesions in the pelvis, which prevents torsion of the ovary; however, rupture can occur in pregnancy due to rapid growth under oestrogen stimulation.
Superficial endometriosis (Sampson’s disease)
Also called non-invasive implants, they are tiny in size with less than 5 mm depth into the surface of the organ involved or peritoneum and are below the resolution of MRI, and hence are not visualized on MRI. However, the presence of small T1-weighted hypertense foci may be the only imaging clue to them [28].
Deep infiltrative endometriosis
Deep infiltrative endometriosis (DIE) is composed of ectopic endometrial glands and stroma with associated dense fibromuscular hypertrophy and eventually fibrosis and adhesion formation between adjacent organs. On MRI, DIE has a spectrum of imaging appearances depending on the relative composition of active glandular elements, fibromuscular stroma, and fibrosis and include cystic, solid-cystic, and solid lesions [28]. The implants’ active glandular elements have 2 appearances on MRI: Cystic or haemorrhagic/proteinaceous. Haemorrhagic or proteinaceous lesions appear as hyperintense on T1W and hypointense on T2W sequence, whereas predominantly cystic elements are hypointense on T1W with the corresponding hyperintensity on the T2W sequence. At the other end of the spectrum, chronic stromal or fibrotic components in the implant are challenging to detect because they appear as linear or spiculated hypointense lesions on all sequences and as linear hypointense bands on T1W and T2W in the case of more subtle adhesions. Therefore, indirect evidence with associated distortion of adjacent organs in more fibrotic lesions is an essential diagnostic clue if present. In severe DIE, dense fibrosis will result in adhesion formation and obliteration of peritoneal recesses. Contrast enhancement is also helpful to make them stand out because they show delayed enhancement [36].
Anterior compartment
MRI is a valuable tool in evaluating DIE of the anterior compartment because its evaluation is not optimal with ultrasonography. Anterior compartment DIE is less frequent than posterior compartment DIE, accounting for ~6% of cases [37]. The vesicouterine space and urinary bladder are the most common sites affected in the anterior compartment.
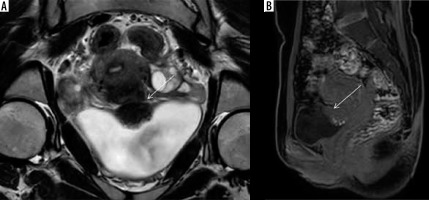
The vesicouterine space is the peritoneal recess between uterus and bladder and is frequently involved in anterior compartment endometriosis resulting in adhesion formation, uterine anteversion, and even complete obliteration in severe cases (Figure 8). Bladder involvement per se is rare, accounting for < 1% of cases of endometriosis; however, it is the most common site of affliction in the urinary tract, followed by distal ureters [38].
Figure 8
Deep infiltrative endometriosis in vesicouterine space with contiguous bladder and uterine involvement. Coronal T2W (A) and sagittal T1 with fat suppression images (B) show obliteration of vesicouterine space with ill-defined stellate-shaped hypointense lesion extending anteriorly into the dome and posterior wall of the bladder and posteriorly involving the anterior wall of the uterus, which appears mildly anteverted. There is also presence of hyperintense foci on T1W images suggestive of haemorrhage within the glandular components
Bladder endometriosis is almost always associated with endometriosis in the vesicouterine space (anterior cul-de-sac); therefore, the dome, posterior wall, and trigone are the most frequent sites of involvement [39]. Involvement is usually multifocal and occurs in 2 patterns: extrinsic and intrinsic, depending on the depth of involvement of the bladder wall. The extrinsic type remains confined to the serosa and does not involve the detrusor muscle layer and mucosa and hence is not evident on cystoscopy. In contrast, the intrinsic type infiltrates into the bladder wall, involves the detrusor muscle layer, and creates mural masses visualized on cystoscopy. If it further involves the mucosa in cases of full-thickness endometriosis, it can even mimic bladder carcinoma [40]. Therefore, while reporting bladder endometriosis, the vital information the surgeon wants for planning is the precise location of the lesion, depth of detrusor invasion, and distance from the ureteral orifice, which will need ureteral reimplantation if involved [41].
Ureteral endometriosis can affect any portion, but distal ureters are most commonly involved and have 2 varieties: extrinsic and intrinsic, similarly to the bladder. The extrinsic variety is more common, appearing as dense hypointense nodules adjoining the distal ureter. As ureters are smaller structures (4-5 mm in diameter), their direct evaluation is limited on MRI due to less spatial resolution, and ureteral dilatation may be the only indirect clue to its presence [42]. It has a strong association with the presence of DIE at other sites, such as endometrioma, uterosacral ligaments, rectovaginal endometriosis more than 3 cm, vagina, bladder, and bowel [43].
Round ligament originates from the anterolateral part of fundus below the fallopian tube, courses anterolaterally, and terminates on labia after passage through inguinal canals. Endometriosis involves the proximal part of the ligament adjacent to the uterus, resulting in shortened, thickened, and nodular appearance [44]. Other sites in the anterior compartment rarely involved are prevesical space, and vesicocervical and vesicovaginal endometriosis, which manifests as the obliteration of the spaces and mass effect on the bladder in the case of prevesical space endometriosis. Urachal involvement is also rarely reported [28,45].
Middle compartment
The middle compartment includes the ovaries, uterus, fallopian tubes, and broad ligament. Apart from the endometrioma, another form of ovarian involvement is adhesions secondary to DIE with resultant medial retraction of the ovaries across the midline behind the uterus in the rectouterine space. Medially displaced ovaries that lie close to each other are referred to as “kissing ovaries”. In addition, medialised ovaries on preoperative imaging are a marker of moderate to severe endometriosis [46].
The uterus can be directly or indirectly affected in DIE. For example, the uterine axis gets distorted and can become retroverted in the posterior compartment disease and anteverted in the anterior compartment because of retraction by dense adhesions. In addition, torus uterinus (slight transverse thickening on the posterior uterine wall at the uterocervical junction) is an attachment site for uterosacral ligaments, typically not visualized in normal people. However, it becomes thickened secondary to endometriotic implants. Finally, the uterus can be involved in uterine serosal plaques on the anterior and posterior surface, which can be very invasive and may mimic focal adenomyosis, especially on the posterior wall [47]. However, uterine DIE is an “outside-inside” process, which can be differentiated from focal adenomyosis, which is an “inside to outside” process, by carefully analysing the endo-myometrial junctional zone, thickened (> 12 mm) and altered in focal adenomyosis and normal in DIE. Therefore, uterine DIE should not be misdiagnosed as focal adenomyosis [48].
Fallopian tube involvement is seen in around 30% of women with endometriosis [49]. DIE implants on the serosa or subserosa of the fallopian tubes are typically not visualized on imaging. However, repeated chronic haemorrhage within the implant and eventual peritubular adhesions cause tubal obstruction and dilation. T1W hyperintense intraluminal signal suggesting hematosalpinx has been seen in only 40% of cases. In the remaining 60% of cases, no T1W hyperintensity is seen in the dilated fallo-pian tube [50]. In addition, unlike endometrioma, T2 shading is not a feature of tubal involvement because endometriotic implants involve the serosal surface of the tube and not the lumen itself [49]. Therefore, hematosalpinx is considered a peculiar finding of endometriosis and may be the only imaging finding that indicates its presence. Differential diagnoses of hematosalpinx include pyosalpinx and fallopian tube malignancy. Fallopian tube malignancies occur in the older age group and show solid enhancing nodules, while pyosalpinx is associated with much fat stranding around the tube with a history of fever and raised leukocyte count.
Similarly to the uterus, involvement of the vagina in endometriosis can be direct or indirect. Directly, the most frequent site is the posterior vaginal fornix, usually secondary to a lesion extending from the retrocervical region. Vaginal endometriosis has a very high association with recurrence and post-surgical rectovaginal fistula formation [51]. Therefore, meticulous evaluation of this region and communication about the exact depth of involvement is essential for presurgical planning. Indirect involvement due to adhesions in the surrounding region will cause angulated and elevated posterior vaginal fornix lying superior to the uterine isthmus.
Posterior compartment
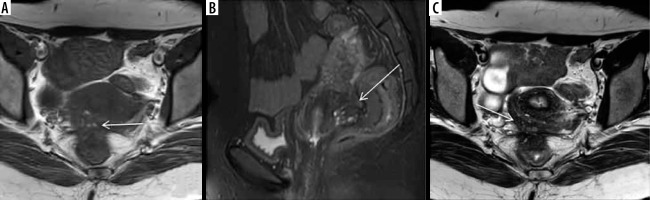
The posterior compartment is the most common site of DIE in the pelvis [20]. It comprises the rectouterine pouch, rectovaginal septum, rectosigmoid bowel, and uterosacral ligaments. The rectouterine pouch or posterior cul-de-sac accounts for most symptomatic cases of endometriosis and is associated with severe disease [52]. It is the most dependent portion of the peritoneal cavity and extends up to the middle third of the vagina and becomes inaccessible on laparoscopy due to its deep position, and it is further problematic in endometriosis because of compartment obliteration. Thus, MRI becomes critical to evaluate rectouterine pouch endometriosis and adhesions. Typical imaging findings include endometriotic implants, either active glandular or chronic stromal/fibrotic, linear hypointense adhesions, and tethering between the uterus and anterior rectum (Figure 9). The rectovaginal septum is an extraperitoneal space between the vagina and lower rectum, extending from the rectouterine pouch to the perineal body. According to the location, lesions can be in the septum (10%) or the posterior vaginal fornix (65%), and there are hourglass-shaped lesions involving the posterior fornix with extension into the anterior rectum [12]. Preoperative mapping of the region is essential because resection of implants predisposes to rectovaginal fistula formation.
Figure 9
Deep infiltrative endometriosis (DIE) in rectouterine pouch. Axial T1W (A), T1W fat suppressed (B), and T2W axial image (C) depict obliteration of rectouterine pouch with ill-defined stellate shaped lesion (white arrows) appearing hypointense on both T1W and T2W images with associated retroversion of the uterus. There is also presence of multiple foci seen within the lesion, appearing hyperintense on T1W and hypointense on T2W images, suggestive of haemorrhagic foci within the ectopic glands
Uterosacral ligaments originate from the torus uterinus, attach posteriorly to the sacrum, and are considered the second most common site after the ovaries in some studies. The proximal third are the most commonly involved sites, presenting as asymmetric shortening, thickening (>9 mm), and nodularity of the involved ligament [53].
Other extrapelvic sites
Extraperitoneal endometriosis
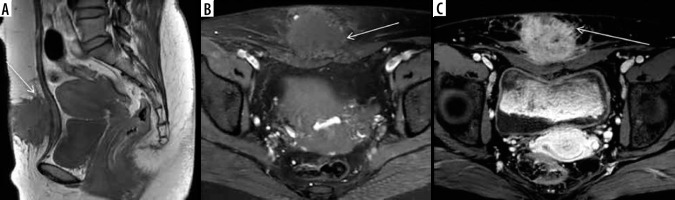
Extraperitoneal endometriosis refers to endometriotic implants outside the peritoneal cavity, and cases have been reported in the literature involving sites like the thoracic cavity, abdominal walls, and pelvic walls. The most common site of involvement is the abdominal wall, which is believed to be due to the direct implantation of endometriotic glands and stroma during the surgery. Women with a history of laparoscopy or caesarean section may present with palpable masses at the incision site, with an incidence of ~15-44% [54]. MRI has a variable appearance, with the majority being hypointense on both T1W and T2W images. The principal differential diagnoses are solid abdominal wall tumours, such as desmoid tumours, but can be differentiated by glandular elements within the lesion showing hyperintensity on T1W sequence or blooming on gradient images (Figures 10 and 11). Another notable location is the pelvic floor at the site of the previous episiotomy scar, which manifests as a palpable nodule in the perianal region with cyclical change in size (Figure 12).
Figure 10
Scar endometrioma in a patient of post laparotomy scar. T1W (A) shows hypointense spiculated ill-defined lesion (white arrows) in the anterior abdominal wall along the subcutaneous plane with hyperintense ectopic endometrial glands on T1 fat suppressed image (B) and post contrast enhancement (C)
Gastrointestinal involvement
Common sites of gastrointestinal tract involvement and corresponding hallmark imaging findings are summarised in Table 2 [55].
Table 2
Gastrointestinal tract involvement in endometriosis
Structured reporting and checklist
The organization of the report in a structured way according to the compartment approach gives a detailed, concise mention of all relevant findings, which helps the surgeon approach the disease effectively and helps the radiologist in deciding the search pattern and reduces the chances of missing findings. An example of such a structured reporting template is provided in Table 3.
Table 3
Structured reporting template for magnetic resonance imaging endometriosis
Conclusions
Endometriosis is a challenging gynaecological disease with non-specific symptoms, and it is often missed and misdiagnosed, resulting in a delay in diagnosis and management, affecting patients’ quality of life. Although laparoscopy is the gold standard for diagnosis, it is an invasive procedure and has limitations in extensive disease with obliteration of spaces. Therefore, imaging becomes critical in its evaluation, and radiologists should not only diagnose endometriosis but should provide a detailed description of all the possible sites of involvement, mapping the entire extent in a compartment-wise manner. Moreover, they should communicate the findings effectively in a structured radiological report emphasizing all the relevant details for effective surgical outcomes and improved patients’ quality of life.