Introduction
Progress in the field of biology and medicine in the XX and XXI century has shifted the epidemiological threats, with civilizational diseases, such as obesity, being recognized as one of the most urgent health challenges to the global population [1,2]. It is estimated that there will be up to 38.1% overweight and 19.7% obese adults by 2030 worldwide, while these numbers in the United States of America (USA) alone will equal 86.3% and 51.1%, respectively [3,4]. In Poland, overweight and obesity is also an important health, social, and economic issue. The World Health Organization (WHO) expects the prevalence of obesity in Poland to reach 28% among men and 18% among women by 2030 [5]. Particularly worrying is the rapidly increasing prevalence of overweight and obesity in young people [6,7].
The role of surgery in the treatment of obesity has been well established over the years, and it is now not only considered the only method resulting in long-lasting significant weight loss effect, but also it has been proven to be more effective than medical therapy in the treatment of comorbidities of obesity [8-10]. The Roux-en-Y gastric bypass (RYGB) and laparoscopic sleeve gastrectomy (LSG) are the most commonly performed surgical bariatric procedures, mainly because of their high success rates and relatively low complication rates. In recent years, the number of LSGs performed worldwide has shown the highest increase rate among other metabolic surgeries [11]. According both to the International Federation for the Surgery of Obesity (IFSO) and the American Society for Metabolic and Bariatric Surgery (ASMBS), LSG has become the most popular bariatric procedure nowadays [12,13]. LSG is perceived as one of the safest bariatric operations. The main advantages of LSG are as follows: a relatively simple surgical technique with no need of anastomosis creation, short learning curve, and low rate of metabolic complications [14].
Because a growing number of surgery clinics are decideing to start a bariatric program, it is highly possible that radiologists, working both in hospital and outpatient clinic environments, will be faced with interpretation of the images of patients after LSG. With the modifications in peri- and postoperative care protocols, the approach to the imaging algorithm is also changing. The purpose of the study is to review available literature and guidelines for imaging after LSG. We will present a surgical technique of LSG performed in our hospital and discuss the methodology and diagnostic capabilities of the main imaging methods – upper gastrointestinal (UGI) series and computed tomography (CT) – with an emphasis on the detection of postoperative complications. Currently, the main method of imaging complications after LSG is CT. UGI is performed less frequently. It can be conducted when leakage or stenosis of the sleeve is suspected or for medical research purposes (to examine the anatomy, shape, or volume of the gastric remnant and its correlation with clinical data) [15,16]. The other methods are auxiliary.
Surgical technique
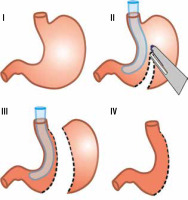
In our hospital, LSG is the most commonly performed bariatric procedure. The first LSG in our hospital was performed in 2011, and since then 50 to 80 operations have been performed annually. According to the “Polish recommendations for bariatric and metabolic surgery” document, the main indications for bariatric surgery are BMI (body-mass index) ≥ 40 or BMI ≥ 35 with coexistence of obesity-specific comorbidities [17]. Each patient is meticulously prepared before final qualification undergoing surgical, cardiological, and anaesthesiological consultations, as well as gastroscopy. Women are also referred for the gynaecological examination. LSG is a restrictive type of bariatric surgery. The operation is performed laparoscopically by 3 surgeons under general anaesthesia. Typically, 5 trocars are used in order to achieve the best exposition. Dissection of the greater curvature of the stomach is performed using a specialist energy device. The width of the sleeve is calibrated using a 36 Fr nasogastric tube, and the resection of the stomach is performed using a linear cutting stapler only (Figure 1). At the beginning of the bariatric program, the staple line was oversewed; however, this step was given up when evidence showed that it does not reduce the risk of leakage [18]. Routinely, UGI was performed on the first day after the operation to rule out leakage, but this approach was abandoned with the end of 2017, when the Early Recovery After Bariatric Surgery (ERABS) protocol was incorporated (Table 1). Along with the introduction of this protocol, early mobilisation of the patients was possible, which shortened the length of hospital stay. It now does not exceed 3 days for patients with uncomplicated postoperative course.
Table 1
Enhanced recovery after bariatric surgery (ERABS) protocol
Imaging following laparoscopic sleeve gastrectomy
Methodology
Upper gastrointestinal
UGI series is the most basic study after LSG. This minimally invasive technique has a long history of being used for the detection of both early and late postoperative complications [19,20]. The examination begins with a single abdominal radiograph in erect position to detect free gas in the peritoneum (which can be noticeable up to 1 week after surgery) and to check the position of drains [21,22]. Then, the patient swallows water-soluble contrast medium [23]. The passage through the upper GI is observed by fluoroscopy, and single X-rays are taken in anterior-posterior (AP), oblique, and lateral projections [24]. Because X-rays of the patient are taken in both vertical and supine positions, care must be taken to ensure that the examination table can withstand the weight of a particularly obese patient [25]. In our Department of Radiology, the UGI study begins with a single abdominal X-ray in standing position (AP projection). Then, the patient is given a glass of 50 mL water-soluble contrast medium to swallow, and the passage of the contrast is observed using fluoroscopy – we examine closely the gastroesophageal junction, sleeve morphology, the staple line, and the passage of contrast medium to the duodenum and further parts of small intestine. Afterwards, the stomach remnant is observed in oblique and lateral projections while the patient is still standing. Next, the patient takes another sip of the contrast medium and is told to lie on their back and on both sides on a bed. After a few minutes, additional X-rays are performed to make sure that there are no signs of leakage. Overhead radiographs following initial fluoroscopy are strongly recommended in this procedure.
Computed tomography
In recent years, CT has been increasingly used as a primary postoperative examination after bariatric procedures for complication detection. The main concern associated with the use of CT is its high radiation dose; however, it has been shown that with a proper protocol an average effective dose of 7.8 mSv is achievable in bariatric patients, which is not much higher than for a UGI study (approximately 6 mSv according to the American College of Radiology) [26].
CT scans from the distal part of the oesophagus to the pelvis are recognized as a default range for patients after LSG to rule out possible complications [21]. The CT protocol depends on the clinical situation. The study always begins with an unenhanced phase. If there is a clinical suspicion of active bleeding or unenhanced phase shows acute haematoma (a fluid collection with attenuation values 40-60 HU), in our opinion it is better to administer intravenous contrast before oral contrast in order to avoid the influence of possible beam hardening artefacts on the interpretation of images. To identify the source of bleeding, arterial phase CT angiographic images are acquired with bolus tracking technique. In other cases, where leak is a primary concern, the patient is required to drink a cup of oral contrast medium on the table (approximately 60 ml). Afterwards, intravenous contrast is administered and portal venous phase images are acquired after 70 s. Delayed phase images were suggested for exclusion of strictures or delayed passage; however, we do not use those routinely because we consider a UGI study to be much more informative in this respect [16].
There is no consensus regarding optimal patient preparation before the CT study. Some authors advocate administration of the solution of sodium bicarbonate and tartaric acid diluted with water or iodinated contrast agent directly before the study in order to expand the stomach remnant [27-31]. When the study is performed for evaluation of the stomach volume, such preparation increases the precision of measurements. For a better quality of the examination, some authors suggest intravenous injection of butylscopolamine prior to scanning [28,30,31].
In uncomplicated cases, CT scans show a tubular gastric sleeve with a staple line along the great curvature. In the area of excised stomach, abundant mesenteric fat as well as small residual pneumoperitoneum (in the early postoperative period) can be found [19,32-34]. No fluid collections should be present.
Morphological and functional assessment
Shape variations
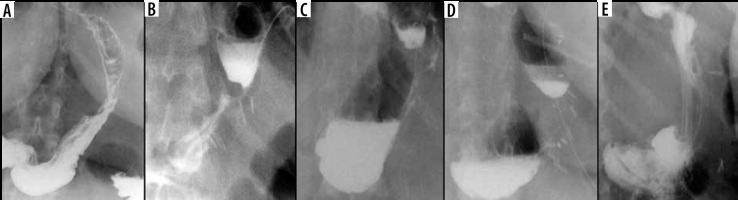
Among the basic information obtainable from the UGI study is a shape characterization of the gastric remnant. Werquin et al. described 5 shape patterns of residual stomach: tubular, superior pouch, inferior pouch, superior-inferior pouch, and pseudodiverticular pattern [35] (Figure 2). The division is based on the staple line curvatures. The most common is tubular pattern. It is represented as a long, round, tube-shaped form with a homogeneous opacification after swallowing the contrast material. The superior pouch refers to a wider superior compartment of the sleeve, near the gastroesophageal junction, which gradually fills with contrast medium and usually creates an air-fluid level. In turn, the inferior pouch represents a wider component in the antral region. The superior-inferior pouch consists of dilatations in both cardiac and antral area. The pseudodiverticular pattern is a diverticular dilatation of the lesser curvature of the gastric sleeve [20,36-38]. The superior pouch pattern may imitate a gastric extraluminal effluent and can generate false positive diagnosis of a leakage [36,37]. Especially small superior pouches with irregular upper or outer contour and small pouches with narrow neck and filiform opacification may mimic leakage [37]. Thus, it is important to be aware of different gastric sleeve shape patterns. Shape variations seem to have an influence on the presence of gastroesophageal reflux syndrome. Occasional regurgitations and vomiting occurring in patients with the tubular shape can be explained by increased intragastric pressure in the upper region of the stomach [20]. On the other hand, the majority of authors report that the superior pouch shape exhibits a stronger connection with the gastroesophageal reflux symptoms when compared to the tubular shape [16,37,38].
Gastric remnant volume
Measurement of the gastric volume after sleeve gastrectomy is not performed routinely, but it can provide valuable data. The gastric volume in the early postoperative period may relate to long-term results in weight loss reduction [39-42]. It can also provide feedback for surgeons who are at the beginning of their learning curve, helping them to create an optimal gastric sleeve. Various methods to evaluate gastric volume on UGI series were described (Table 2). One of them compares the gastric sleeve shape to an ellipsoid, and the volume can be estimated by using the mathematical formula for this figure (V = 4/3 × π × a × b ×c, where V = gastric volume, a, b, c = the ellipsoid’s 3 different radiuses) [42,43]. Two views in perpendicular projections are needed in this approach. Another option is built upon the similarity of the gastric sleeve to a cylinder and uses the following formula: V = π × r2 × h, where V = gastric volume, h = height, and r = radius [39,40]. The height of the cylinder is measured along the long axis of the gastric sleeve, from the cardia to the pylorus. The radius is estimated from the arithmetic mean of 3 maximum widths (measured in the upper, the medium, and the lower part of the residual stomach) divided by 2. In an alternative approach, the volume of the stomach remnant is calculated using the sum of 2 formulas: for a cylinder and for a truncated cone (V = 1/3 π(R2 + r2 + R r) ×h, where V = gastric volume, R = maximum radius, r = minimum radius, and h = height) [41]. The abovementioned formulas are not highly precise because gastric sleeve shapes are not ideal geometric figures [40]. More precise measurements are possible using multidetector CT with oral contrast agent and effervescent agent, because radiological workstations enable semiautomatic volume measurements both on multiplanar and 3D displays [28,44,45]. The optimal gastric volume is still being debated; however, most publications suggest gastric sleeve volumes oscillating between 50 and 120 ml [40,41,44,46].
Table 2
Different approaches to calculate gastric remnant volume
| Shape | Ellipsoid | Cylinder | Cylinder + Truncated cone |
|---|---|---|---|
| Projection |  |  |  |
| Formula | V = 4/3 × π × a × b × c | V = π × r2 × h | V = π × r2 × h + 1/3 × π × (R12 + R22 + R1 × R2) × H |
Table 3
Computed tomography (CT) classification of leak after laparoscopic sleeve gastrectomy (LSG) [63]
Passage
The UGI series is the only technique allowing close evaluation of the contrast passage through the gastric remnant in a real time. Goitein et al. identified 2 patterns of contrast medium passage after LSG: a rapid, uninterrupted contrast transit through the gastric sleeve from the gastroesophageal junction through the pylorus to the duodenum (passage < 30 seconds) and a delayed contrast flow to the duodenum (passage > 30 seconds) [47,48]. Patients from the first group were found to have better fluid tolerance in the early postoperative period and were discharged earlier than the second group [47]. Prolonged contrast transit to the duodenum in the early postoperative period most often results from oedema of the pylorus wall [34]. Similarly, Pomerri et al. categorized patients into 2 groups: fast passage group – when gastric emptying takes < 1 minute; and slow passage group – when gastric emptying lasts ≥ 1 minute [42]. They observed that the patients with fast gastric voiding had a higher weight loss 1 year after LSG with percentage of excess BMI loss (%EBL) > 50% as compared to the patients with slow passage, who often had an inadequate weight loss (%EBL < 50%) at all follow-up time points [42].
Detection of complications
Leak
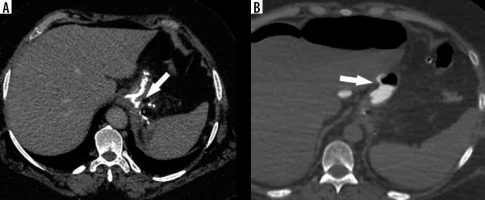
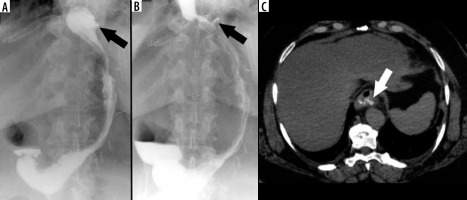
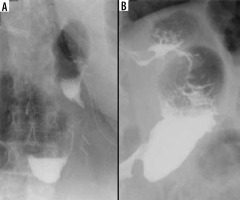
The reported incidence of leakage after LSG ranges between 1.09 and 5.3%, and it is a very dangerous complication that can lead to peritonitis, sepsis, formation of an abscess or a fistula, and even death [49-51]. Clinical manifestations of this particular complication are unspecific and include abdominal pain, fever, and tachycardia [23,52]. Leaks after LSG generally occur in the early postoperative period; however, they may occur up to 8 days after the surgery. According to the surgical literature, 2 types of leak are recognised: type I or subclinical (small, local, without diffusion through a fistula, and no presence of methylene blue after oral administration in any of the abdominal drains) and type II or clinical (leakage with early septic manifestation and extensive dissemination to the pleural or abdominal cavity with presence of methylene blue in any of the abdominal drains) [52-54]. Radiographically, leakage is defined as a flow of the contrast medium through the staple line into the peritoneal cavity [55]. On UGI series leakage can present either as small effluents or more defined collections of extraluminal contrast medium [19,56]. However, it should be emphasised that UGI has a low leak detection rate. While most papers have reported high specificity (up to 100% in selected papers), the sensitivity is unacceptably low, ranging from 0% to 33% [57-60]. If there is a suspicion of leak, either clinically or on the UGI study, a CT scan should be performed to help with a swift and confident diagnosis establishment (Figure 3). CT has a much higher leak detection rate (up to 86%) [49, 61]. On CT, leak can present as extravasation of oral contrast medium into the peritoneum and sometimes into drains, fluid collection, or free fluid (Figure 4). When there are perigastric fluid collections, the absence of contrast medium extravasation does not preclude an active leak [62]. Nedelcu et al. created a CT classification of leak after LSG [63]. This classification recognizes 4 types of leak based on the size and location of fluid collections: type I – < 5 cm in the left upper quadrant of the abdomen, type II – > 5 cm in the left upper quadrant of the abdomen with two subtypes: a – negative leak visualisation or b – positive leak visualisation, type III – diffuse abdominal collections, type IV – pleural (thoracic) collections; the modifiers indicate the leak location in relation to the part of the gastric sleeve: S – superior, M – middle, I – inferior (Table 2). Nedelcu et al. created an algorithm for the management in different types of leak: type I leaks, which are clinically well tolerated, may be treated with starvation diet and intravenous antibiotics, and controlled with gastroscopy or CT scan. When conservative treatment is not effective enough or when any other type of leak is diagnosed, laparoscopic drainage should be performed; in type IV pleural drainage is necessary [63]. In persistent gastric leaks, treatment is usually tailored individually to each patient. Laparoscopic reoperation such as re-sleeve gastrectomy, conversion to Roux-en-Y gastric bypass, or total gastrectomy may be performed. Endoscopic management strategies include clipping, stenting, endoluminal vacuum or J-tube placement [64,65] (Figure 5).
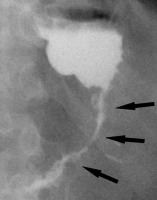
Figure 3
Example of a false positive leak result in upper gastrointestinal series. Unusual surplus of contrast medium nearby the gastroesophageal junction (black arrows on A and B). On computed tomography scan (C), there is a pseudodiverticulum in this location (white arrow)
Bleeding
Postoperative bleeding usually occurs at the level of the gastric staple line, most often within the first 72 hours after surgery, and can be intra- or extraluminal. Intraluminal bleeding usually presents with haematemesis or melena stools. Signs of extraluminal bleeding include drops in serum haemoglobin level, tachycardia, and hypotension. Besides of the staple line, common origins of extraluminal bleeding are injuries to the spleen, liver, or abdominal wall at trocar entry sites [66,67]. In a majority of cases, conservative treatment is sufficient. Persistent active bleeding is an indication for surgical intervention or intravascular treatment depending on local expertise [68]. CT angiography is the method of choice for the detection of active bleeding; however, haemodynamically unstable patients should undergo surgical or intravascular intervention as soon as possible.
Infection
Infection is a possible early complication after LSG. Abscesses usually result from a leak and are most often located near the staple line in the left upper quadrant, most frequently in the subphrenic space [36]. Typical clinical manifestations include fever, abdominal pain, nausea, and vomiting. If there is a clinical suspicion of an infection, a CT scan of the abdomen should be performed. Portal venous phase offers the best means of detection of pathological fluid collections. Therapeutic drainage of the abscess can be conducted under CT guidance [34].
Splenic infarction
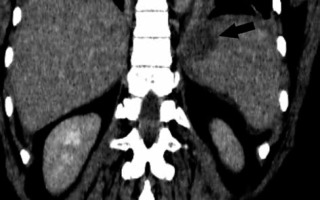
There is a wide range of anatomic variants of the arterial supply of the spleen. Anastomotic connections between the blood supply of the stomach and the spleen are often present [69,70]. During the LSG procedure, mobilization of the gastric fundus along with ligation of the short gastric vessels is necessary, which may cause insufficient splenic perfusion, most often in the upper pole of the spleen. The infarction may be asymptomatic or manifest like any other possible complication after LSG (fever, abdominal pain, tachycardia with increased inflammatory markers) and can lead to splenic abscess formation [71]. The diagnosis is established by conducting an angio-CT study of the abdomen (Figure 6). Gas within the infarcted area suggests abscess formation. In uncomplicated cases, conservative treatment is usually sufficient.
Stricture
Another potential complication following LSG is gastric sleeve stricture. Clinical manifestations consist of dysphagia, stomach pain, nausea, and vomiting. The most vulnerable part of the stomach is the area of incisura angularis [36,72]. In the early postoperative period stenosis is usually caused by oedema and in a majority of cases is reversible. Stenosis may develop when the sleeve is too narrow, most commonly due to a technical error during the operation. Strictures can also be a side effect of oversewing of the staple line or could develop from fibrosis situated on the site of a previous leak, fistula, or haematoma [19,34] (Figure 7). Due to its dynamic character, UGI series is more effective in stricture detection than CT. The passage of swallowed contrast medium could be delayed or stopped [16]. In addition, gastric dilatation proximal to the stenotic segment is usually observed. When the stricture is at the level of gastroesophageal junction, the lower part of the oesophagus will be dilated [37]. The treatment of strictures, especially in the early postoperative period, is usually started in a conservative manner with proton-pump inhibitors and nasogastric tube placement or by endoscopic balloon dilatation [21,37]. Insertion of a stent is sometimes required [16,36]. In some cases surgical management, such as seromyotomy or conversion to RYGB (Roux-en-Y gastric bypass), is necessary [73].
Torsion of the gastric sleeve is a dangerous complication that can present similarly to stricture. It occurs when the anterior and the posterior wall of the stomach are not of identical length from the lesser curvature [21]. UGI shows dilatation of the proximal part of the gastric sleeve and delayed or stopped passage of the contrast medium, while the staple line exhibits a twisted course [19]. The management of this complication is with endoscopic or surgical intervention.
Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) is a prevalent condition in morbidly obese patients. LSG can cause exacerbation, de novo appearance or reduction of its symptoms. Most of the studies suggest surgical technique and anatomical changes of the stomach as potential cause of the appearance of gastroesophageal reflux symptoms after LSG. Alleged reasons of GERD symptoms are reduction of lower oesophageal sphincter pressure, probably due to postoperative alteration of the angle of His, and enlargement or development of hiatal hernia [36,74,75]. Another reason could be gastric remnant stenosis near incisura angularis as a result of increased gastric fundal pressure [72]. As mentioned before, a superior pouch shape of gastric sleeve predisposes to gastroesophageal reflux [16,37,38].
Dilatation
Gastric sleeve dilatation is a late complication occurring usually 2-3 years after the surgery. Gastric remnants have a tendency to extend progressively, which may lead to weight regain and may be a reason to perform revisional bariatric surgery [72,74]. A wide sleeve formation during a primary surgery is a predisposing factor for later dilatation. Other contributing factors include excessive pressure generated from consuming large meal volumes, repeated vomiting, or distal stricture of the stomach remnant [76]. Increase of gastric remnant volume and loss of its tubular shape may be observed on the UGI series [56] (Figure 8).
Figure 8
Upper gastrointestinal study. A) Early postoperative examination. B) Stomach dilatation 2 years after the surgery
To sum up, UGI and CT are fundamental imaging techniques after LSG. For a long time, in multiple surgical centres, UGI was performed after every operation, but following numerous studies and controversies, UGI is no longer considered to be a routine examination [23,57,59,60]. According to the ERABS protocol, a liquid diet should be initiated without conducting UGI examination only a few hours after LSG [77]. UGI’s low sensitivity in leak detection and high cumulative financial cost are perceived as significant drawbacks [57-60]. CT has a high sensitivity in leak detection and additionally visualizes other complications invisible on radiography, such as abscesses, active bleeding, or splenic infarction. UGI is still useful when functional disturbances are suspected, being very effective in the demonstration of strictures, dilatations, and abnormal passage. Gastric remnant volume calculation, performed either with UGI series or CT, is usually employed in scientific research.
Conclusions
There is no consensus on routine imaging after LSG in leak detection. Nowadays a lot of bariatric centres implement the ERABS protocol, which excludes routine use of radiological examinations in the postoperative period. If a patient experiences worrisome symptoms after the operation, CT is a method of choice in complication detection in the early postoperative period.